


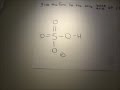













H 2SO4 sulfuric acid will donate an H + in solution to form H 3O+ hydronium. The remaining H SO− 4 would be the conjugate base of this dissociation. I hope this was helpful. Click here👆to get an answer to your question ️ Conjugate base of H2SO4 is: H2SO4 is the chemical name for sulfuric acid, and the conjugate base is hydrogen sulfate. Even though HSO4 is also an acid, it can either be a conjugate acid or a conjugate base depending on context. A conjugate base is not necessarily a basic molecule. The given species H2SO4 is an acid so it is suppose to donate a proton and after donating a proton it will be HSO4 - H2SO4 <-----> HSO4- + H + Now, HSO4-is a base since it has the ability to accept a proton but it is a conjugate base to H 2 SO 4 since it is formed by the H2SO4 after donating a proton. How to solve: What is the conjugate base of H2SO4? By signing up, you'll get thousands of step-by-step solutions to your homework questions. You... what is the conjugate base for HClO4. what is the conjugate acid for ClO4^-what is the conjugate base for H2SO4 A conjugate base is what is left when an acid loses a proton (H+), for example the conjugate base of sulfuric acid (H2SO4) is the bisulfate ion (HSO4-). Favorite Answer. The conjugate base will have one less H atom than the acid H2As04-. So the conjugate base will be HAs042-. There is another negative charge because a proton (aka positive charge... What will the conjugate bases for the following Bronsted acids: HF , H2SO4 HCO3 - and NH4 + asked Jun 2, 2018 in Chemistry by Golu (106k points The species H2O, HCO3^-, HSO4^- and NH3 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base. asked Feb 12, 2020 in Chemistry by SurajKumar (66.2k
[index] [1540] [1679] [7182] [769] [9027] [1835] [6097] [1243] [6740] [1238]
Here I am just answering one of my hw problems. I will try to post as much hw as I can This is for an OCHEM CLASS. All the answers provided are checked by th... 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One... Chemistry: Learn more at: http://www.pathwaystochemistry.com/chemistry-qa/videos/conjugate-acid-base-pairs/ About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Determine acid/base ratio of a buffer - Duration: 12:43. Peter Klappa 38,107 views. 12:43. Using the HH equation to calculate partial charges on amino acids - Duration: 5:26. The content of this video is designed to accompany the 12th edition of "Chemistry The Central Science" by Brown, Lemay, Bursten, Murphy, and Woodward. The ti... Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together... Introduction to conjugate acids and bases. Created by Sal Khan.Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is...
Copyright © 2024 top100.playrealmoneytopgame.xyz